AI chat is no longer just about faster answers or conversational tone. Search.com, a division of Public Good, has launched a generative AI search…
Funding, regulations top the challenges Africa’s healthcare startups face [Sponsored]

A shortage of funding, overcoming regulations and accessing laboratory space. These are just some of the challenges that African tech startups in the health-care sector face.
Despite these, four startups are forging ahead in a bid to make an impact in the continent’s health-care sector.
Earlier this year the four — Akili Labs (from South Africa), Bluewave Insurance (Kenya), Chekkit Technologies (Nigeria) and Epilert (Tunisia) — took part in a joint startup programme implemented by Merck Accelerator and tech entrepreneurship initiative Make-IT in Africa in partnership with HYBR (see this story).
The four now aim to explore and validate the potential for collaboration in the health sector in partnership with Merck.
Four startups with the help of the Merck Accelerator are forging ahead in a bid to make an impact in Africa’s healthcare sector
Ventureburn asked the four about the main challenges they are facing and about the impact they have made inspire of these hurdles.
‘Deep tech investment a hurdle’
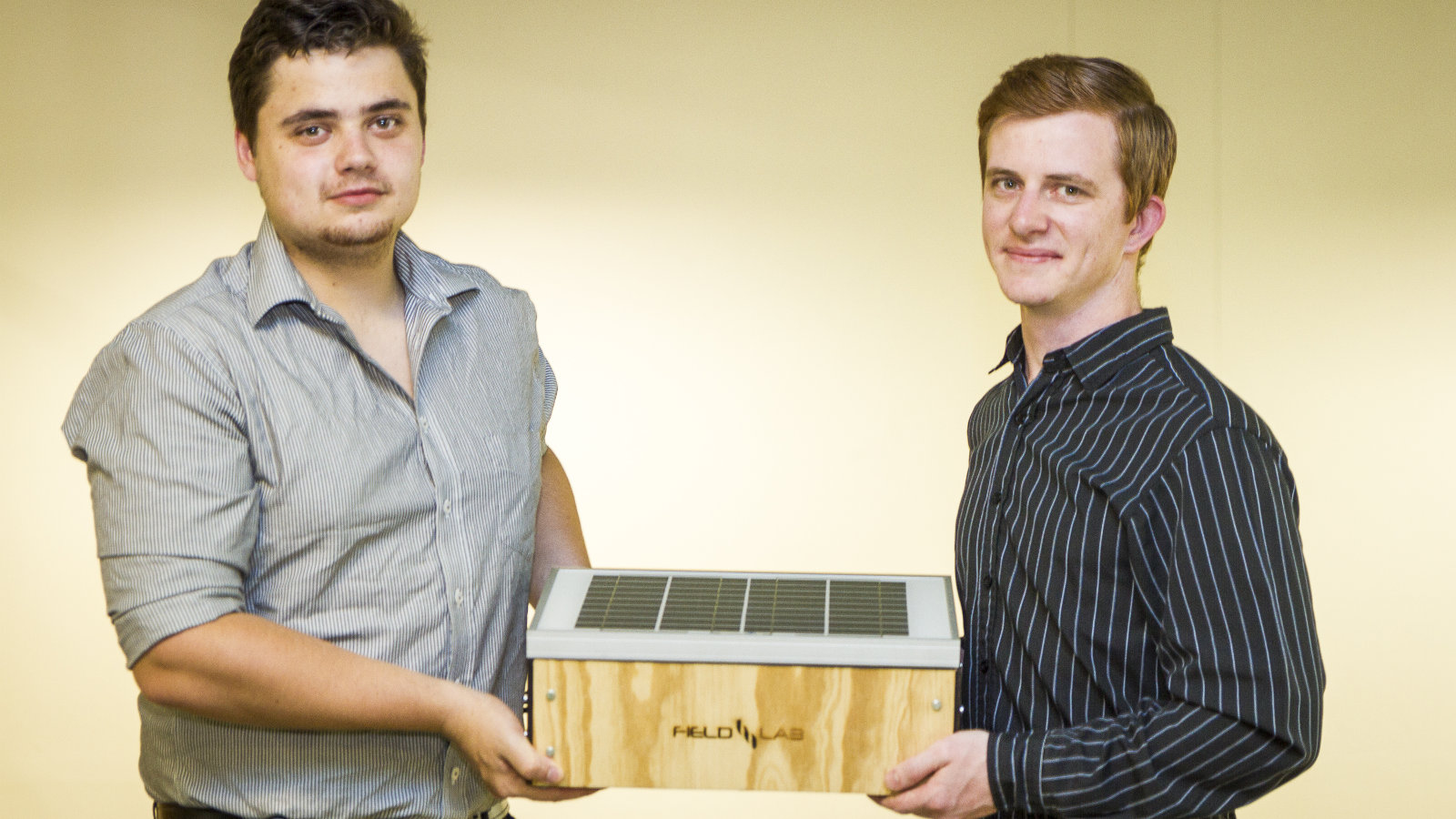
Akili Labs co-founder and CEO Charles Faul (pictured above, right with co-founder Lucas Lotter) believes one of the single biggest challenges biotech startups face in Africa is accessing lab space as well as the cost of setting up their own laboratory.
“(The lack of an) Appetite for deep tech in investment in Africa can also be a large hurdle,” adds Faul.
Faul’s biomedical startup specialises in medical diagnostic technology. He founded the Pretoria-based company in 2015 with COO Lucas Lotter and CMO Farhan Pervazi.
The startup initially developed low-cost laboratory equipment to meet the demand for advanced custom laboratory equipment using Internet of Things (IoT) hardware and additive manufacturing methods such as fused deposition modelling (FDM) and stereolithography (SLA) 3D printing.
“This then later brought us into the field of medical diagnostics when we investigated Ebola point of care diagnostics while at Rhodes University.
“We found a severe lack of appropriate point of care systems and started designing our technology to solve this problem using novel technology we developed for the detection of pathogens (viruses or bacteria) in blood samples,” says Faul.
The startup is currently developing MobiMed, an integrated handheld point-of-care (POC) molecular diagnostic system.
“We are currently performing validations on HIV, however we can’t disclose where and who, but we can say it is with a partner that is very well skilled in running clinical trials and validation studies on pharmaceutics and devices in Africa.”
“Once we launch we will be able to improve access to laboratory-grade testing at point of care which will greatly improve patient outcomes,” says Faul.
He points out that the Akili Labs is developing partnerships with a number of South African organisations these include Savant Technology Incubator, The Innovation Hub, Hamamatsu and Rhodes University’s BioSENS Research Group.
Akili Labs is currently raising a seed round which it has earmarked for trials and to advance its development. Since it launched four years ago startup has raised R1.1-million which includes seed funding from Savant and prize money from the GAP BioSciences competition.
Faul says the startup is looking at expanding to neighbouring African countries and has plans to enter the US market via potential American partners in research and development (R&D) and business development.
He says Akili Labs has “greatly benefited” from Merck’s programme in that the startup has been able to expand its network, with better visibility of the market across Africa.
“We are hoping to get a major partnership, which may reduce the time to get to market for the kind of technology we develop and improve our position in the market,” says Faul.
‘Adapting to large insurance companies’
Nairobi-based insurtech Bluewave Insurance is looking to make health services accessible to all, by making it easier for low-income earners to get medical insurance.
For as little as $3 a month, Bluewave’s micro-insurance product Imarisha enables anyone with a simple basic feature phone to get access to a $1000 life, $1000 disability and $100 hospital cash.
BlueWave was founded in 2016 by insurance expert Adelaide Odhiambo (pictured above).
She was later joined by early-seed stage investor and COO Mandeep Birdi who has worked with mobile network operators around the world. He helped Indian telco Airtel launch micro-insurance products across eight different markets in Africa.
Odhiambo says one challenge is that the startup is that because it operates in the insurance sector, the startup must adhere to the strict structures and processes of traditional insurance companies, which can cause delays.
“Startups pride themselves in speed to market and innovation. But our biggest challenge has been that of adapting to large insurance companies. It is our vision to have our own insurance license,” says Odhiambo.
She says the startup has plans to launch a new product every three months through its partnership with an unnamed large micro-finance institution which will act as a distribution partner.
Through its participation in Merck’s programme, she says Bluewave is looking to benefit from the data and information the company has on healthcare.
“Merck, being one of the leading global science and technology companies, will be such a critical partner in helping us to better position our health capitation products,” she says.
By joining this programme she says the startup is hoping to be supported by leading global players to see how best we can be able to scale up our products, our proposition to improve healthcare globally.
To date, Bluewave has raised $300 000 in funding from Samurai Incubate, 1to4 and from Birdi who happens to be the startup’s first angel investor.
Last year the startup won the Nairobi leg of Swiss emerging markets startup competition Seedstars, and with it the opportunity to pitch for up to $500 000 in investment at the competition’s global summit held in Lausanne, Switzerland in April.
The startup is currently busy with a Series-A round and aims to raise $1-million to aid its international expansion.
Odhiambo says the startup recently entered Malawi, is scoping the market in Democratic Republic of Congo (DRC), is fully operational in Kenya and has also received some interested to work in the South East Asia Pacific markets.
Says Odhiambo: “We have a vision to be in 10 African markets by the year 2030”.
‘State agencies must come to party’
Dare Odumade, the CEO of Lagos-based Chekkit says his startup aims to gain sustainable market share but that this is dependent on a lot of factors which include — but are not limited to collaborations with government agencies.
“We believe there’s a need for a platform for identifying high impact or growth ventures through which governments in West Africa could partner with those startups that have direct value to offer regulatory bodies which will aid current inefficient processes,” says Odumade (pictured above, middle with two co-founders).
Chekkit develops anti-counterfeiting solutions that track and authenticate products from the producer to the consumer.
The startup was founded last year by Odumade, Adebola Oyenuga, Jida Asare and Oluwatosin Adelowo (pictured above, left with Odumade and Oyenuga).
Odumade says the idea for the startup came about after a patient of Asare, who is a trained and licensed pharmacist, died after consuming counterfeit drugs unknowingly.
“The fact is we generally purchase drugs from neighbourhood pharmacies we trust but these trust metrics are based on proximity to our location and the reality is the pharma supply chain in Africa is heavily fragmented such that pharmacies often sell a fair mix of high quality and sub-standard drugs for the purpose of maximising
profit,” says Odumude.
Chekkit has developed two products that leverage its anti-counterfeit technology — namely its consumer intelligence (CI) solution for product anti-counterfeiting, brand protection and real-time market insights and its product supply chain tracking (SCT) solution, which is still in private beta.
The CI product, which was launched in November last year, has since been used by three large fast moving consumer good (FMCG) companies in Nigeria including cosmetics brand Nivea.
Odumade says the startup’s solution has not only protected these brands but have also increased customer loyalty and provided them with real-time insights on consumer purchase patterns as related to specific products. He says the product has seen over 30 000 consumers authentications carried out via USSD.
The startup now plans to launch a mobile app this quarter to scan Chekkit QR labels which he says have multi-security features and cost much less than USSD security PIN authentication.
“The new Chekkit App will allow consumers authenticate by scanning Chekkit tamper-proof QRs labels on products and report bad experiences directly to producers and regulators,” he says.
Although Chekkit is based in Nigeria, it also operates in Ghana and is expanding to Zimbabwe through local partnerships. The startup is also planning a gradual expansion into the rest of the continent.
Odumade and his three fellow co-founders have bootstrapped the startup since launch, with some of its funding comprising grants, loans and revenue.
The startup has raised grants from the Facebook Startup Accelerator, British Council, Tony Elumelu Foundation and ARM Daayta Challenge.
The startup is currently raising a seed round which it hopes to close by the end of November and will be used to cover operational costs and to finance the Chekkit’s technology.
With its participation in the Merck programme, Odumade says Chekkit is looking to benefit from new opportunities to work with Merck.
“The programme will give us access to launch a proof of concept with Merck for example helping to track Merck’s product supply chain and consumption behaviours, market dynamics, estimate demand and ultimately improve Merck’s business presence in Africa while using our learnings to continuously improve our products
and offerings,” he says.
Odumade says by 2024 the startup aims to reduce deaths caused by counterfeit pharmaceuticals and FMCG products annually by 70% and save companies more than $1.5-trillion in losses caused by counterfeit goods.

‘Health regulation is a big challenge’
Motivated by a sister who is epileptic, Tunisian Firas Rhaeim (pictured above) decided to launch Epilert, a solution to tackle the problems that his family faced with the disorder.
Rhaeim, who has a background in engineering, along with COO Harroun Moula and business developer Amine Riahi came up with Epilert, a wearable bracelet with integrated biosensors for epilepsy and other neurological disorders.
The device is currently in the last stages of prototyping and is set to launch clinical trials at the Al Razi Hospital in November and if all goes well Epilert will be delivered to customers from February next year. The startup has so far raised $300 000 in investment.
Rhaeim says the medtech startup is currently working with partners that include Analog Device, Arrow, Onetech and Al Razi Hospital.
The programme, he says, will help Epilert leverage Merck’s expertise and connect data from the startup’s wearable device to enhance treatment within Merck therapeutic areas.
Rhaeim says Epilert’s biggest challenge has been around meeting the regulatory requirements.
“Currently we are working on obtaining the necessary certification such as FDA (Federal Drug Authority approval) then we aim to be available in all Africa and the US by mid 2021, covering more than 14 million customers in need of our device,” he says.
Rhaeim says Epilert will provide hope to a lot of people when it’s launched, adding that the innovation is designed to give a peace of mind for families and epileptics.
Selection Days and MEDICA conference
According to Herve Kubwimana, Manager of the Merck Accelerator in Africa, “we cannot wait to welcome all these four incredible teams during the next phase of the programme in Germany to attend our Selection Days and MEDICA conference. We believe in their potential to collaborate with Merck and we are excited to help them
achieve this”.
Dr Jan Schwaab, Head of Tech Entrepreneurship Initiative Make-IT in Africa states, “we are looking very much forward to have the four teams in Germany for the final phase of the programme.
“We want to leverage our extensive network of partners for the benefit of the start-ups to further support digital innovations for sustainable development. It is of importance to not just to support them to achieve their short-term and long-term goals, but also to build a bridge between different ecosystems and strengthen the collaboration.”
To learn more about the Merck Accelerator programme and the Merck Innovation Center, click here.
To learn more about Tech Entrepreneurship Initiative ‘Make-IT in Africa’ click here.
Read more: Four healthcare startups selected for Merck, Make IT in Africa programme [Sponsored]
Read more: Want to work with Merck? Here’s how your startup can stand out [Sponsored]
Read more: Merck is looking to partner with 20 healthtech startups in new programme
Read more: SA startup Akili Labs to exhibit affordable mobile lab at US tech conference
Read more: Kenyan insurtech Bluewave takes top honours at 2018 Seedstars Nairobi
This article is sponsored by Merck.
Featured image: rawpixel via Pixabay